How Does Fluorine React With Ethene
Another type of MO the π orbital may be formed from two p-orbitals by a lateral overlap as shown in part A of the following diagram. Acetic acid fluoro- sodium salt.

Solved 14 Use The Following Enthalpies Of Reaction To Chegg Com
An unusual aminoferrocene AF agent 3 d is reported which does not react with reactive oxygen species ROS in contrast to usual AF agents e.

. 147 Addition Polymerisation A polymer is a compound with very long carbon chains made up of monomer units. A fluorine atom as a substituent on carbon 2 of. When the hydrogen halides react with alkenes the hydrogen-halogen bond has to be broken.
The reaction happens at room temperature in the presence of organic peroxides or some oxygen from the air. How many water molecules H2O are needed to react with 8 ethene molecules C2H4 to produce ethyl alcohol molecules C2H5OH. Pyridines have been shown to mediate the release of ethene from a terphenyl supported 13-dioxo-2-phospholane.
The reaction is a simple addition of the hydrogen bromide. Although we have ignored the remaining p-orbitals their inclusion in a molecular orbital treatment does not lead to any additional bonding as may be shown by activating the fluorine correlation diagram below. UNSATURATED HYDROCARBONS Have double bonds React with aqueous bromine turning the mixture from orange to colourless.
What are the parts of an atom. A fluorine atom as a substituent on carbon 2 of. The hydrogen ensures that the resulting alkenes and cycloalkenes subsequently react with hydrogen to form saturated compounds.
May react with water may give off toxic gases and may be capable of detonation or explosion. The way in which a compound will react is determined by a particular characteristic of a group of atoms and the way they are bonded eg. Study Guide and Solutions Manual to Accompany TW.
The hydroxyl groups react with the other compound often consisting of a polymeric isocyanate such as a trimer of 16-diisocyanatohexane hexamethylene diisocyanate. The bond strength falls as you go from HF to HI and the hydrogen-fluorine bond is particularly strong. Such a compound is known as a crosslinker for it produces on reaction with the resin a three-dimensional structure similar to the polyurethane formed from a polyol and an isocyanate.
Other molecule has a N O or F atom present that possesses one or more nonbonding electron pairs. For example with ethene. Double C-C bond C-OH group.
Alkylation In a refinery alkylation refers to the alkylation of alkanes for example 2-methylpropane isobutane with alkenes in the presence of a strong acid catalyst such as hydrofluoric acid or sulfuric acid. Fryhle Scott A. Because it is difficult to break the bond between the hydrogen and the fluorine the addition of HF is bound to be slow.
Recall that electro-negativity decreases as atom moves further away from fluorine on the periodic chart. One molecule has a hydrogen atom attached by a covalent bond to an atom of nitrogen oxygen or fluorine AND. Wastewater from the reactor vent gas scrubber in the production of ethylene dibromide via bromination of ethene T.
Ethene is a chemical compound used in plants to stimulate the ripening of fruits and the opening of flowers. 2If there is more than one atom type in the molecule put the most metallic or least electronegative atom in the center. 3Arrange the electrons so that each atom contributes one electron to a single bond between each atom.
Alkenes react very slowly with oxygen to produce traces of organic peroxides so the two possible conditions are equivalent to each other. This generates a dioxophosphorane dimer which is an oxygen analogue of Lawesson. Is a polymer produced from ethene by addition polymerization.
The way in which a compound will react is determined by a particular characteristic of a group of atoms and the way they are bonded eg. Double C-C bond C-OH group. Protons neutrons and electronsThe protons and neutrons are packed together into the center of the atom which is called the nucleus and the electrons which are very much smaller whizz around the outsideWhen people draw pictures of atoms they show the.
Graham Solomons Craig B. Most atoms have three different subatomic particles inside them. Ethene is a chemical compound used in plants to stimulate the ripening of fruits and the opening of flowers.
Snyder Jon Antilla.
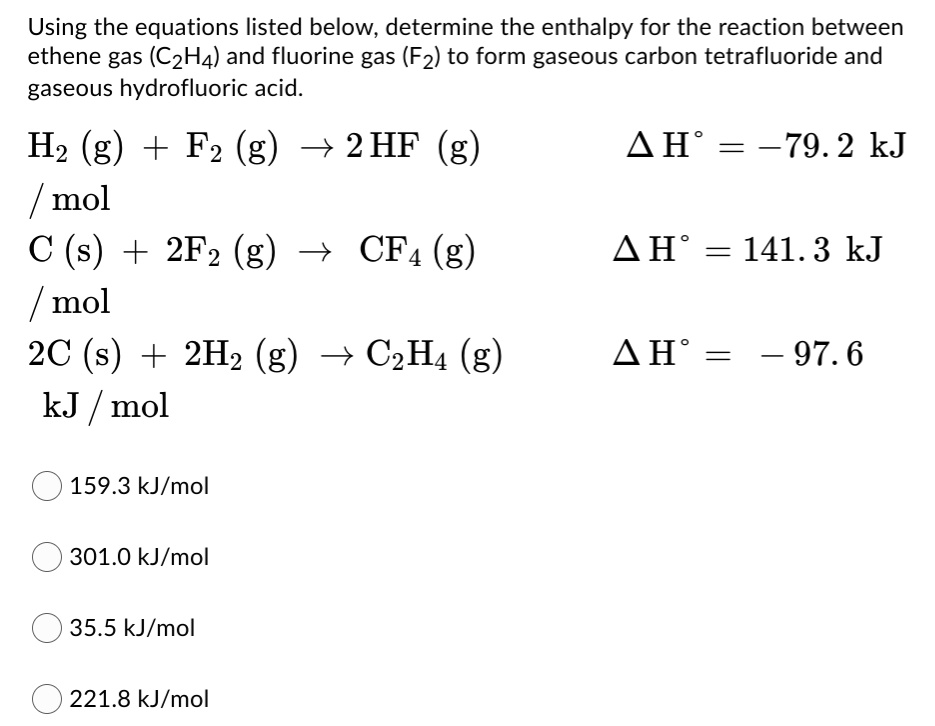
Solved Using The Equations Listed Below Determine The Enthalpy For The Reaction Between Ethene Gas C2h4 And Fluorine Gas F2 To Form Gaseous Carbon Tetrafluoride And Gaseous Hydrofluoric Acid Hz G Fz

Hydrocarbons David Martin City And Islington College A Hydrocarbon Is An Organic Compound Consisting Entirely Of Hydrogen And Carbon Ppt Download
Addition Reaction Of Alkenes With Halogens Easy Exam Revision Notes For Gsce Chemistry

Welcome To The Oc Organic Chem In Notes Ppt Video Online Download
Comments
Post a Comment